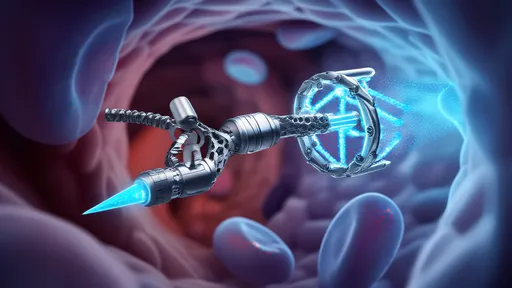
In the rapidly evolving field of nanotechnology, DNA-based nanomachines have emerged as a groundbreaking tool with the potential to revolutionize medical science. These microscopic devices, engineered from the very building blocks of life, are being hailed as molecular scalpels capable of performing precise interventions at the cellular level. Unlike conventional surgical tools, DNA nanomachines operate with unparalleled precision, targeting specific cells or molecules without disrupting surrounding tissues.
The concept of DNA nanomachines leverages the natural properties of DNA molecules—their ability to self-assemble, their programmability, and their biocompatibility. Scientists have harnessed these traits to create structures that can walk, rotate, and even perform computational tasks. One of the most promising applications lies in their ability to act as molecular surgeons, delivering drugs, repairing damaged DNA, or selectively destroying harmful cells.
Recent advancements have demonstrated the potential of these nanomachines in cancer treatment. Traditional chemotherapy often attacks healthy cells alongside cancerous ones, leading to severe side effects. DNA nanomachines, however, can be designed to recognize and bind exclusively to cancer cells. Once attached, they release therapeutic payloads or trigger apoptosis, the programmed death of the targeted cell. This level of specificity could drastically reduce the collateral damage associated with current treatments.
Beyond oncology, DNA nanomachines are being explored for their ability to manipulate individual molecules. Researchers have developed devices that can cut and paste DNA strands with atomic precision, offering new avenues for gene editing. Unlike CRISPR, which relies on bacterial proteins, these nanomachines are entirely synthetic, reducing the risk of immune responses. This could pave the way for safer and more efficient gene therapies for genetic disorders.
The development of DNA nanomachines is not without challenges. One major hurdle is ensuring their stability in the complex environment of the human body. Enzymes, pH fluctuations, and other cellular components can interfere with their function. Scientists are addressing this by engineering more robust structures and protective coatings. Another challenge is scalability—manufacturing these devices in quantities sufficient for clinical use remains a significant obstacle.
Despite these challenges, the progress in this field has been staggering. Early-stage trials have shown promising results, and the pace of innovation shows no signs of slowing. As researchers continue to refine these molecular tools, the line between science fiction and medical reality grows increasingly blurred. The day when doctors wield DNA nanomachines as routinely as scalpels may not be far off.
Ethical considerations also come into play with such powerful technology. The ability to manipulate life at the molecular level raises questions about unintended consequences and potential misuse. Regulatory frameworks will need to evolve alongside the technology to ensure its safe and ethical application. Public dialogue and interdisciplinary collaboration will be crucial in navigating these complexities.
Looking ahead, the integration of DNA nanomachines into mainstream medicine could herald a new era of precision healthcare. From targeted cancer therapies to personalized gene editing, the possibilities are vast. As this technology matures, it may transform not only how we treat diseases but also how we understand the very fabric of life. The journey from laboratory curiosity to clinical staple is fraught with challenges, but the potential rewards make it a pursuit worth undertaking.

By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025